The Effect of Sous-vide Cooking on the Physical & chemical Properties of Meat Products
Heating meat causes extensive structural changes in its protein composition. At the level of three-dimensional structure, meat proteins denature and form aggregates when heated. At the molecular level, changes in primary structure (amino acid residues) in cooked meat include protein pulping, modification of aromatic residues and the formation of Maillard reaction products. The identification of these modifiers is critical to determine the thermal processing mechanism of meat and to better control the nutritional and functional properties of the product.
1.1 Changes in protein structure
High temperatures will lead to protein denaturation, which greatly reduces the water-holding capacity of meat. In the process of muscle contraction, water, soluble protein and some fat will be excreted. The lateral contraction of myofibrils is the main reason for the reduction of moisture content in the cooking process. However, as the heating intensity increases and the heating process continues, the denaturation and longitudinal shrinkage of collagen will lead to further losses. The limited longitudinal shrinkage which occurs may be part of the reason that the process produces fewer losses than other cooking methods that use higher temperatures.
The structures of different proteins changed sequentially at different temperatures. First, from 40 to 52.5°C, sarcoplasmic proteins and myofibrillar proteins were denatured, resulting in a slow loss of fluid from the muscle fibers to the extra-muscular space; second, between 52.5 and 60°C, the water loss of the muscle fibers is getting faster and faster, reaching a maximum speed and extent at about 59°C, but the length of the muscle is still not completely shortened, which may be mainly due to the thermal contraction of collagen in the muscle at about 58°C. At 64 to 94°C, lateral and longitudinal contractions of muscle fibers become obvious, and this process is accompanied by increased thermal processing losses, and thermal contraction of intramuscular, perimuscular, and extra muscular collagen reaches the greatest extent. Finally, long-time cooking results in partial or complete gelatinization of extra muscular collagen, followed by perimuscular and intramuscular collagen. The process of gelatinization involves the structural decomposition of the collagen protein helix into random helices structure, thereby forming a gel that is different from the natural collagen and can be soluble in warm water.
The effect of increasing heating time on the change in pork diameter was relatively small. Therefore, there was no significant difference in fiber diameter with increasing heating time from 3 hours to 20 hours for pork sealed by a vacuum sealer heated at 53, 55, 57 or 59°C. Mutton and beef also show a similar pattern. Myofibrils are dynamic protein net structures composed of several interacting different proteins including actin and myosin that provide stability to protein complexes and muscle structure. When the heating temperature is higher than 53°C, complete denaturation occurs, and this denaturation is also affected by the heat preservation time. Promeyra and others proposed a model of heated myofibrillar protein aggregation. Moderate heating can cause myofibrillar proteins to form fibrillar aggregates (heating at 80°C for no more than 20 minutes, or heating below 80°C), while excessive heating converts fibrillar aggregates into random aggregates (heating at 80°C for more than 20 minutes, or heating above 80°C).
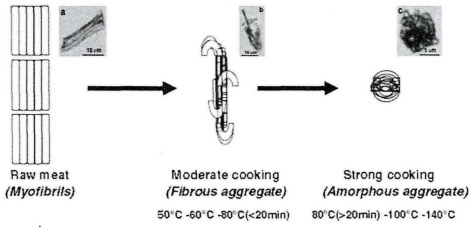
Figure 2 The schematic diagram of protein aggregation due to heating
1.2 Hydrolysis of protein
People always believed that during mild heat treatment, proteolysis is an auxiliary mechanism for the thermal degradation of collagen. Christensen and others detected residual cathepsins B and L in the broth of meat heated at low temperatures for a long time. Residual cathepsin activity in cooked pork (semitendinosus and longissimus) increased at temperatures from 48 to 58℃, while it decreased when holding time increased from 5 hours to 17 hours. Kristensens and others proposed the possibility of thermal activation of proteases during heat treatment and found that calpain remained active for 1 to 3 hours at 40°C based on similar findings. Cathepsin B and L could remain active at 55℃, but only for about 1 hour at 70℃. Cathepsin can destroy the stability of natural collagen, and can also degrade collagen thermally into peptides, which can be further degraded into smaller peptides by other enzymes. Veroniquel and others found in the research process that the thermal processing of meat products will affect the sensitivity of myofibrillar proteins to proteases, and the increase or decrease of the digestion rate depends on the nature of the protease and the thermal processing time and temperatures.
1.1 Changes in protein structure
High temperatures will lead to protein denaturation, which greatly reduces the water-holding capacity of meat. In the process of muscle contraction, water, soluble protein and some fat will be excreted. The lateral contraction of myofibrils is the main reason for the reduction of moisture content in the cooking process. However, as the heating intensity increases and the heating process continues, the denaturation and longitudinal shrinkage of collagen will lead to further losses. The limited longitudinal shrinkage which occurs may be part of the reason that the process produces fewer losses than other cooking methods that use higher temperatures.
The structures of different proteins changed sequentially at different temperatures. First, from 40 to 52.5°C, sarcoplasmic proteins and myofibrillar proteins were denatured, resulting in a slow loss of fluid from the muscle fibers to the extra-muscular space; second, between 52.5 and 60°C, the water loss of the muscle fibers is getting faster and faster, reaching a maximum speed and extent at about 59°C, but the length of the muscle is still not completely shortened, which may be mainly due to the thermal contraction of collagen in the muscle at about 58°C. At 64 to 94°C, lateral and longitudinal contractions of muscle fibers become obvious, and this process is accompanied by increased thermal processing losses, and thermal contraction of intramuscular, perimuscular, and extra muscular collagen reaches the greatest extent. Finally, long-time cooking results in partial or complete gelatinization of extra muscular collagen, followed by perimuscular and intramuscular collagen. The process of gelatinization involves the structural decomposition of the collagen protein helix into random helices structure, thereby forming a gel that is different from the natural collagen and can be soluble in warm water.
The effect of increasing heating time on the change in pork diameter was relatively small. Therefore, there was no significant difference in fiber diameter with increasing heating time from 3 hours to 20 hours for pork sealed by a vacuum sealer heated at 53, 55, 57 or 59°C. Mutton and beef also show a similar pattern. Myofibrils are dynamic protein net structures composed of several interacting different proteins including actin and myosin that provide stability to protein complexes and muscle structure. When the heating temperature is higher than 53°C, complete denaturation occurs, and this denaturation is also affected by the heat preservation time. Promeyra and others proposed a model of heated myofibrillar protein aggregation. Moderate heating can cause myofibrillar proteins to form fibrillar aggregates (heating at 80°C for no more than 20 minutes, or heating below 80°C), while excessive heating converts fibrillar aggregates into random aggregates (heating at 80°C for more than 20 minutes, or heating above 80°C).

Figure 2 The schematic diagram of protein aggregation due to heating
1.2 Hydrolysis of protein
People always believed that during mild heat treatment, proteolysis is an auxiliary mechanism for the thermal degradation of collagen. Christensen and others detected residual cathepsins B and L in the broth of meat heated at low temperatures for a long time. Residual cathepsin activity in cooked pork (semitendinosus and longissimus) increased at temperatures from 48 to 58℃, while it decreased when holding time increased from 5 hours to 17 hours. Kristensens and others proposed the possibility of thermal activation of proteases during heat treatment and found that calpain remained active for 1 to 3 hours at 40°C based on similar findings. Cathepsin B and L could remain active at 55℃, but only for about 1 hour at 70℃. Cathepsin can destroy the stability of natural collagen, and can also degrade collagen thermally into peptides, which can be further degraded into smaller peptides by other enzymes. Veroniquel and others found in the research process that the thermal processing of meat products will affect the sensitivity of myofibrillar proteins to proteases, and the increase or decrease of the digestion rate depends on the nature of the protease and the thermal processing time and temperatures.

